Class 11 Chemistry Solutions For Filling Up Of Electrons In Different Orbitals
Question 1. Write de Broglie equation for microscopic particles.
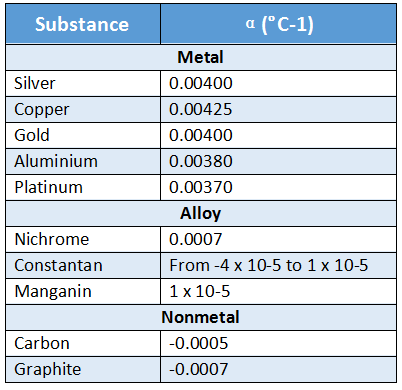
Answer: \(\lambda=\frac{h}{p}\) wavelength p = momentum of particle (mv).
Question 2. What is the relation between wave nature and particle
nature of moving particles?
Answer: Wave nature \(\propto \frac{1}{\text { particle nature }}\)
Question 3. Derive a relation between kinetic energy and de Broglie wavelength associated with a moving electron.
Answer:
We know, the kinetic energy (E) of the particle moving with velocity v, is given by, \(E=\frac{1}{2} m v^2\) or, 2E = mv²
or, 2mE = m²v²
⇒ \(m v=\sqrt{2 m E}\)
Question 4. What happens to the de Broglie wavelength associated with a moving particle if its velocity is doubled?
Answer:
The de Broglie wavelength reduces to half its initial value
⇒ \(\text { [as } \left.\lambda=\frac{h}{m v} \text {, or } \lambda \propto \frac{1}{v}\right]\)
Class 11 Chemistry Electron Configuration Solutions
Question 5. A hard-struck cricket ball does not produce waves. Why?
Answer:
Due to the large size of the cricket ball, its mass is large and hence its wavelength is negligible.
Therefore \(\lambda \propto \frac{1}{m}\)
Question 6. Two particles P and Q are moving with the same velocity, but the de Broglie wavelength of P is thrice that of Q. What do you conclude?
Answer:
⇒ \(\text { Since } \lambda^{\circ} \frac{1}{m}, \lambda_P \propto \frac{1}{m_P} \text { and } \lambda_Q \propto \frac{1}{m_Q}\)
⇒ \(\frac{\lambda_P}{\lambda_Q}=\frac{m_Q}{m_P}, \text { or } \frac{m_Q}{m_P}=\frac{\lambda_P}{\lambda_Q}=\frac{3}{1} \text { or, } m_Q=3 \times m_P\)
Therefore Mass of Q is Thrice that of P.
Question 7. Compare the wavelengths of a molecule of each O2 and CO2, travelling with the same velocity.
Answer:
⇒ \(\text { Since } \lambda \propto \frac{1}{m} ; \quad \lambda_{\mathrm{O}_2} \propto \frac{1}{32 \mathrm{u}} \text { and } \lambda_{\mathrm{CO}_2} \propto \frac{1}{44 \mathrm{u}}\)
[ V Molar mass of O2& CO2 are 32u & 44u respectively]
Thus, the wavelengths of a molecule of each O2 and CO2 traveling with the same velocity is in the ratio 11:8
Question 8. Why is the de Broglie wave termed a matter wave?
Answer:
Since de Broglie wave is associated with fast-moving tiny material particles, it is also known as matter wave. The wavelength of such waves depends on the mass and velocity of the particles.
Question 9. Write the mathematical expression for Heisenberg’s uncertainty principle-
Answer:
⇒ \(\Delta x \cdot \Delta p \frac{h}{4 \pi},\) where Ax and Ap are uncertainties in the determination of exact position and momentum respectively.
Question 10. For which particles is the uncertainty principle applicable?
Answer:
Heisenberg’s uncertainty principle applies to tiny subatomic particles like electrons, protons, neutrons, etc.
Filling Of Electrons In Orbitals Class 11
Question 11. Is there any significance of Heisenberg’s uncertainty principle in our daily life?
Answer:
In our daily life, we deal with objects of ordinary size. So the uncertainties in their position and momentum are very small as compared to the size and momentum of the n- n-object respectively. So, such uncertainties may be neglected. Thus, the uncertainty principle has no significance in our daily life.
Question 12. Why does Bohr’s model contradict Heisenberg’s uncertainty principle?
Answer:
According to Bohr’s theory, negatively charged particles (electrons) inside an atom revolve around the nucleus in well-defined orbits having a fixed radius.
In order to balance the nuclear attractive force, electrons must move with a definite velocity. However, according to the uncertainty principle, it is impossible to determine simultaneously the exact position and the momentum (or velocity) of a microscopic particle like an electron. Thus, Bohr’s model contradicts Heisenberg’s uncertainty principle.
Question 13. Explain why the uncertainty principle is significant only for
subatomic particles, but not for macroscopic objects.
Answer:
The position of a subatomic particle can be located accurately by illuminating it with some electromagnetic radiation.
The energy of the photon associated with such radiation is sufficient to disturb a subatomic particle so that there is uncertainty in the measurement of the position and momentum of the subatomic particles. However, this energy is insufficient to disturb a macroscopic object.
Question 14. Why is it not possible to overcome the uncertainty of Heisenberg’s principle using devices having high precision?
Answer:
Heisenberg’s uncertainty principle has no relation with the precisions of measuring devices.
- AVe know that the subatomic particles are very tiny and thus cannot be seen measured even under a powerful microscope; To velocity or to locate the position of the subatomic particles, they are illuminated (struck, hyprotons) with suitable electromagnetic radiation.
- Hence, the precision of measuring devices is not possible in overcoming Heisenberg’s uncertainty principle.
Question 15. Is Heisenberg’s uncertainty principle applicable to a stationary electron? Explain
Answer:
It is not applicable. Since the velocity of a stationary electron is ‘zero’, (v = 0), its position can be located accurately.
Wbbse Class 11 Chemistry Solutions
Question 16. Write Schrodinger’s wave equation, indicating the significance ofthe notations used.
Answer: Schrodinger’s wave equation is based on the dual nature (wave and particle) of electrons.
Question 17. What is the basis of Schrodinger’s wave equation?
Answer: Schrodinger’s wave equation is based on the dual nature (wave and particle) of electrons.
Question 18. Schrodinger’s wave equation does not give us any idea about which quantum number.
Answer: Spin quantum number (s).
Question 19. What is the physical significance of & and ifr2?
Answer: The wave function has no physical significance, while 4r2 gives the probability density i.e., the probability of finding the electron at any point around the nucleus.
Question 20. Write Schrodinger’s wave equation in the briefest possible form.
Answer: The briefest form of Schrodinger’s wave equation is, Hi// = Eip, where H is known as the Hamiltonian operator.
Question 21. How many radial nodes are present in (i) 3s -orbital and (11) 2p -orbital?
Answer:
Radial nodes of 3s -orbital =n-l-1 = 3-0-1 = 2
Radial nodes of 2p -orbital = n- Z-1 = 2-1-1 = 0
Question 22. How many radial nodes and planar nodes are present in 3p -orbital?
Answer:
No. of radial nodes =n-Z-1 = 3-1-1 = 1
No. of planar nodes =1=1
Total no. of nodes = n- l = 3- l = 2
Question 23. How many nodal planes are present in 5d –orbital.
Answer: Number of nodal planes in 5d -orbital = 2
Question 24. Write the expression for radial distribution Junction.
Answer: RDF = 4nr2i/f2(r)dr
Aufbau Principle Class 11 Solutions
Question 25. Calculate the number of radial nodes and planar nodes in 2 orbital. x2-y
Answer: No. ofradial nodes =n-l-1 = 4-2-1 = 1
No. of planar nodes = 1 = 2
Question 26. What do you mean by the Acceptable values of e and Corresponding wave functions that are obtained by solving the Schrodinger wave equation for h-atom?
Answer:
No, atomic orbitals do not possess a sharp boundary. This is because the electron clouds are scattered to a large distance from the nucleus. The density of electron clouds decreases with increasing distance from the nucleus but theoretically, it never becomes zero.
Question 27. Does atomic orbitals possess a sharp boundary? Explain.
Answer:
No, atomic orbitals do not possess a sharp boundary. This is because the electron clouds are scattered to a large distance from the nucleus.
The density of electron clouds decreases with increasing distance from the nucleus but theoretically, it never becomes zero.
Question 28. What will be the sign of 2p along an axis on the two opposite sides ofthe nucleus?
Answer:
The sign of 2p along an axis will be opposite on the two opposite sides ofthe nucleus
Question 29. What will be the values of ip2 , ip2 value of – 0 ? ,>x py
Answer:
When r = 0 , the value of i//2 , i/r„ and i/r, is zero (0).
Question 30. In which direction the value of(l), (ii) (III) ifr2 is the highest? Px
Answer:
- At the negative and positive direction of the x -x-axis.
- At the negative and positive direction of y -y-axis.
- At the negative and positive direction of the z-axis.
Hund’s Rule And Pauli Exclusion Principle Class 11
Question 31. Why do p -p-orbitals possess directional properties?
Answer: The angular part of the wave function of p -p-orbital depends on the value of 6 and <p. Thus, p -p-orbitals possess directional properties.
Question 32. Why s -orbital does not possess directional properties?
Answer: The angular part of the wave function of s -orbital does not depend on 6 and <p.
As a result, a symmetrical distribution of electron density occurs with increasing distance from the nucleus. Thus, s -the orbital is spherically symmetrical and does not possess directional properties.
Question 33. In which direction the value of/zÿ is zero?
Answer: Along the x,y and z-axis
Question 34. In which direction the value of y2 is the highest?
Answer: Along the x and y-axis.
Question 35. What do you mean by ‘doughnut’?
Answer: The two lobes of d -the orbital are distributed along the z-axis and a sphere is situated with the nucleus at its centre. This sphere is called a ‘doughnut’
Question 36. How many angular nodes are present in dÿ -orbital?
Identify them.
Answer: Two angular nodes are present (xz -plane and yz -plane).
Question 37. In which direction is the value ofdÿ, is zero?
Answer: Along z -the z-axis.
Question 38. How many angular nodes are present in dÿ Identify them.
Answer: Two angular nodes are present (they pass through the
origin and lie at an angle of 45° with the xz and zipline and themselves lie perpendicular to each other)
Question 39. How many angular nodes are possible for an orbital?
Answer: ‘V number of angular nodes are possible (where Z = azimuthal quantum number).
Electron Configuration Class 11 Solved Examples
Question 40. Does the number of angular nodes of an orbital depend on the principal quantum number?
Answer: No, it depends only on the azimuthal quantum number.
Question 41. How many angular nodes are present in s -s-orbital? Indicate the subshells present in the M -M-shell. How many orbitals are present in this shell?
Answer: s -orbital does not possess any angular nodes because the value of the angular wave function cannot be zero in any direction.
Question 42. Indicate the subshells present in the M -M-shell. How many
orbitals are present in this shell?
Answer: In the case of M-shell, the principal quantum number, n = 3. The values of azimuthal quantum no. Z are’ 0 ‘, ‘ I’ and ‘2’.
This means that the M-shell contains three subshells, namely, ‘p’ and ‘ d’. For each value of‘ Z ’, the magnetic quantum number ‘ m ’ can have 2Z + 1 values. Therefore M-shell contains 2Z+ 1 orbital.
Question 43. Write the values of the azimuthal quantum number ‘l’ in the third energy level and(it) 3d -subshell of an atom.
Answer:
In the third energy level, the principal quantum number n = 3
Values of azimuthal quantum no., ‘ Z ’ are 0, 1 and 2.
For any d -subshell, 1 = 2.
Question 44. What is the maximum number of electrons that can be accommodated in the subshell with 1 = 3?
Answer:
For every value of’ Z ‘, ‘ m ’ can have 2Z + 1 values.
Since For Z = 3, m can have 2Z+ 1 values, i.e., 2×3 + 1 = 7 values. Therefore, the number of orbitals in the given shell = 7.
The maximum number of electrons that can be accommodated in these orbitals =2×7 = 14 [v each orbital can accommodate a maximum of 2 electrons].
Class 11 Chemistry Important Questions On Electron Configuration
Question 45. What is the maximum number of electrons that can be accommodated in an orbital with m = +3?
Answer:
Each value of the magnetic quantum number ‘m’ indicates only one orbital and each orbital can accommodate a maximum of 2 electrons.
This means that the orbital Indicated by m = +3 can accommodate a maximum of 2 electrons.
Question 46. Which of the following subshells have no real existence?
- 2d
- 3f,
- 4g and
- 5d
Answer:
In the case of d -subshell, 1 = 2. In the second shell ( n = 2), the values of l are 0 and 1. So there cannot be any d -d-subshell In the third shell. Therefore, we can say that there Is no real existence of a 2d subshell.
In case of/-subshell, l = 3 . In the third shell (n = 3), the values of l are 0, 1 and 2. Since there cannot be any /-subshell In this shell, there is no real existence of 3/- subshell.
ln case of g -subshell, I = 4. In the fourth shell (n = 4), the values of l are 0, 1, 2 and 3. Since there cannot be any g subshell in this shell, there is no real existence of a 4g subshell.
In the case of d -subshell, 1 = 2. In the fifth shell (n = 5), the values of l are 0, 1, 2, 3 and 4. Therefore 5d sub-shell has real existence.
Question 47. How many quantum numbers are needed to designate an orbital? Name them.
Answer:
Three quantum numbers are needed to designate an orbital, namely, ‘ n ‘ l’ and ‘ m
Question 48. Identify the subshells denoted by the following:
- n = 4,l = 2
- n = 5, l = 3
- n = 6, l = 4
Answer:
- 4d
- 5f
- 6g
- 4s
Question 49. An electron is described by magnetic quantum no.m = +3. Indicate the lowest possible value of ‘n ’for this electron. (tv) n = 4,1=0
Answer:
- For an electron having magnetic quantum number m = +3, the lowest possible value of azimuthal quantum number ‘ l’ would be 3 and for an electron having 1 = 3, the lowest possible value for ‘ n’ would be 4.
- In other words, the lowest possible energy level (‘n’) that the electron would occupy is the 4th shell (n = 4).
- For an electron having magnetic quantum number m = +3, the lowest possible value of azimuthal quantum number ‘ l’ would be 3 and for an electron having 1 = 3, the lowest possible value for ‘ n’ would be 4.
In other words, the lowest possible energy level (‘n’) that the electron would occupy is the 4th shell (n = 4).
Question 50. Which quantum number is to be mentioned to distinguish between theefitfrpns__present in the K -shell?
Answer:
For k-shell (n = 1), l = 0 and m = 0. This indicates that K-shell has only one orbital and this orbital can accommodate a maximum of 2 electrons having spin quantum no., ‘s’ with values +1/2 and -1/2.
So, to distinguish between the two electrons in the K-shell, it is important to indicate their spin quantum numbers.
Question 51. Write the vdltt&Wfthe magnetic quantum number for the ‘3d ’-orbitals.
Answer: For 3d orbitals, 1 = 2. Hence the values of the magnetic quantum no., ‘ m’ are +2, +1, 0, -1, -2.
Question 52. Write the values of n, l and m for 3p -subshell.
Answer: For 3p -orbitals, n = 3 , l = 1 and m = +1 , 0, -1 . Hence, 3p -subshell has 3 orbitals.
Class 11 Chemistry Board Exam Solutions
The values of and ‘ m ‘ for these orbitals are as follows:
- n = 3, l= 1 , m = +1
- n = 3,l=l,m = 0
- n = 3, l = 1 , m =-1
Question 53. Which of the following two orbitals is associated with a higher energy?
- n = 3, l = 2, m = +1
- n = 4, l = 0, m = 0
Answer:
The algebraic sum of n and / determines the energy of a given subshell. The higher the value of (n + l), of an orbital, the higher its energy. Thus, the orbital with n = 3, l= 2 is associated with a higher energy.
Question 54. Is there any difference between the angular momentum of 3p and 4p -electrons?
Answer:
For any p -subshell, 1=1. The angular momentum of an electron depends on the values of all and is independent of the values of Angular momentum, \(L=\sqrt{l(l+1)} \frac{h}{2 \pi}.\).
Since 1=1 for both the p -subshells (3p and 4p), there is no difference in the angular momentum ofthe electrons occupying those subshells
Question 55. 4f-subshell of an atom contains 10 electrons. How many of Write the expression for the orbital angular momentum of a revolving electron.
Answer:
The orbital angular momentum of the electron, ‘U is given by: L \(\sqrt{l(l+1)} \times \frac{h}{2 \pi}\)
Question 56. Write the expression for the orbital angular momentum of a
revolving electron
Answer: The orbital angular momentum of an electron, ‘U is given by: I = \(=\sqrt{l(l+1)} \times \frac{h}{2 \pi}.\)
Question 57. Mention the sequence in which the following orbitals are filled up by electrons: 3d and 4p.
Answer:
The energy of a given subshell is determined by the algebraic sum of’ and ‘Vue., n + 1.
11 the ‘n +l’ values of any two subshells are equal, then the electron enters the one with lower’ n ’. In the 3d -subshell, n = 3 , l = 2 n + l = 3 + 2 = 5 In the 4p -subshell, n = 4, 1=1:.
B +1 =4 +1 = 5 Since for the 3d -subshell, n = 3 which is lower than that of 4p where n = 4, the electron first enters the 3d -subshell.
Question 58. What is the maximum number of Ad -electrons with spin quantum number s =?
Answer:
For a 4d -subshell, n = 4, 1 = 2 and m = +2, +1,0, -1,-2. This implies that there are 5 orbitals in this sub-shell that can accommodate a maximum of 10 electrons.
5 of these electrons have s = +| and the remaining 5 have -i . Hence maximum number of 4d -electrons with 2 i spin quantum no., s = -1/2 is 5.
Question 59. Is it possible for atoms with even atomic numbers to contain unpaired electrons?
Answer:
Atoms with even atomic numbers can have unpaired electrons. This is in accordance with Hund’s rule which states that the orbitals within the same subshell are at first filled up singly with electrons having parallel spin before pairing takes place.
For instance, in the case of a carbon atom (atomic number 6 and electronic configuration: ls22s22p1x2p1y2p0z ), there are two unpaired electrons
Question 60. Write the electronic configurations of Cu and Cr -atoms.
Answer:
Electronic configuration of 2gCu
ls22s22p63s23p63d104s1
Electronic configuration of 24Cr :
ls22s22p63s23p63d54s1
Class 11 Chemistry Electron Configuration Solutions
Question 61. Write the electronic configurations of Fe2+ and Cu+ ions.
Answer:
Electronic configuration of Fe2+ (atomic number =26):
ls22s22p63s23p63d6
Electronic configuration of Cu+ (atomic number = 29 ):
1s22s22p63s23p63rf10
Question 62. Which of the following has a maximum number of unpaired electrons?
- Mn2+
- Fe2+
- Cu2+
- Cr
Answer: The following are the electronic configurations of the given ions and atoms:
- Mn2=: ls22s22p63s23p63d5
- Fe2+: ls22s22p63s23p63d9
- Cu2+: ls22s22p63s23p63d9
- Cr: ls22s22p63s23p63d54s1
Question 63. Calculate the number of unpaired electrons in the N -atom.
Answer:
Electronic configuration of (atomic no. =7): ls22s22p3
According to Hund’s rule, the 3 electrons in the 2psubshell occupy the three p -p-orbitals (px, py, pz) singly.
Hence, the no. of unpaired electrons present in N = 3.
Question 64. How many electrons of the Ne -atom have clockwise spin?
Answer:
Electronic configuration of Ne (atomic number = 10)
Each of the pair of electrons present in each ofthe Is, 2s, 2px, 2py, and 2pz orbitals have a clockwise spin and the other, an anti-clockwise spin. Therefore no. of electrons of Ne-atom having clockwise spin = 5.
Question 65. Why does an electron pair in an orbital have an opposite spin?
Answer:
If a pair of electrons with parallel spin are present in the same orbital then they will repel each other.
Question 66. Write the names and symbols of an atom, a cation and an anion with the electronic configuration Is2.
Answer:
Atom: Helium (He);
Cation: Lithium-ion (Li+),
Anion: Hydride ion (H- ).
Filling Of Electrons In Orbitals Class 11
Question 67. Which quantum numbers specify the size and the shape of electronic orbital?
Answer: The size of an electronic orbital is determined by the principal quantum number (n) and the azimuthal quantum number (I) determines the shape of an electronic orbital.
Question 68. Write down the values of the quantum numbers of the electron in the outermost shell of sodium.
Answer: The electron present in the outermost shell of sodium is identified as 3s1. Its principal quantum number n = 3, azimuthal quantum number 1 = 0, magnetic quantum number, m = 0 and spin quantum number, s = +1/2.
Question 69. How many nodes are there in 3s -orbital?
Answer: The spherical shell (or region) inside the s -s-orbital where electron density is zero is called the node. In the case of 3s orbital, there are two such spherical shells where the electron density is zero.
So 3s -orbital has two radial nodes but no angular node (1 = 0). So the total no. of nodes is 2. [No. of nodal surfaces =n- 1, where n is the principal quantum number]
Question 70. How many nodal points are there in 3p -orbital?
Answer: In a p -orbital the electron density, at the point where the two lobes meet is zero.
This point is called the nodal point of the p-orbital. So each of the three 3p -orbitals (viz., px, py and pz ) has only one nodal point.
Question 71. Indicate principal and azimuthal quantum numbers for the subshells:
- 4s
- 5d
- 2p
- 6
Answer:
- n=4, l=0
- n=5,l=2
- n=2,=1
- n=6, l=3
Question 72. Which is the lowest principal energy level that permits the existence of off-subshell?
Answer: For f-subshell the value of azimuthal quantum number Z is 3. So the lowest principal energy level that permits the existence of an f -subshell is the fourth shell (i.e, N -N-shell)
Wbbse Class 11 Chemistry Solutions
Question 73. An element (symbol M) has 26 protons in the nucleus. Write the electronic configuration of M2+ and M3+.
Answer:
26M: ls22s22p63s23p63d64s2
26m2+: ls22s22p63s23p63d6
26m3+ : ls22s22p6 3s2 3p63d
Question 74. There are 8 electrons in the 3d -subshell of an atom. Among these, what will be the maximum number of electrons with similar spin? What is the number of odd electrons?
Answer: Electronic configuration of 3d -subshell
Maximum number of electrons with the same spin = 5.
Number of odd electrons in that atom = 2.