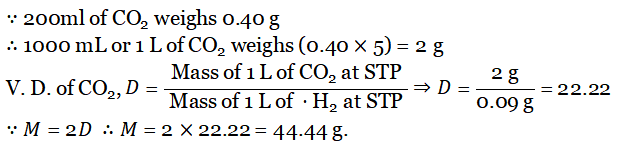WBBSE Chapter 3 Stoichiometric Equations Chemical Reactions
Stoichiometry is a kind of calculation that chemists use. We know that a chemical equation is a complete description of a chemical reaction by using symbols and formulae of all reactants and products.
Reactant 1 + Reactant 2→Products.
Stoichiometry helps us to find out how much reactance is used up or what product is produced in a chemical reaction.
It deals with the calculation of various quantities (no. of mol/mass/volume) of reactants and products. We need two things to do in stoichiometry,
A balanced chemical equation is a concise description in which the no. of atoms of each reactant/product remains the same on either side,
Wbbse Class 10 Physical Science Notes
Some measured values of the chemicals involved in the reaction. These values are used to find out whatever (no. of mol/mass/volume) is unknown.
WBBSE Notes For Class 10 Physical Science And Environment
(Is. 1.2. Conservation of mass in chemical reactions Stoichiometry works on the basis of the law of conservation of mass. Scientist Lavoisier proposed this law in the year 1774.
It states that in chemical reactions matter (or mass) is neither created nor destroyed. That is the total mass of reactants before the reaction = the total mass of products after the reaction.
If you have a certain no. of H atoms at the start of a reaction, you will have the same no. of H-atom at the end of the reaction.
As an example, we can consider the following reaction:
Wbbse Class 10 Physical Science Notes

However, for accurate measurement, it is seen that every chemical reaction is associated with the emission or absorption of energy (heat).
This was successfully explained by the great physicist Albert Einstein. He explained that mass and energy are interconvertible according to the relation E = mc²,
Wbbse Class 10 Physical Science Notes
Where the amount of mass disappears to produce an equivalent amount of energy and is the speed of light in a vacuum.
For example, in a reaction 1000J of energy would be released due to a loss in mass \(m=\frac{E}{c^2}=\frac{1000 \mathrm{~J}}{\left(3 \times 10^8 \mathrm{~m} / \mathrm{s}\right)^2}=1 \cdot 11 \times 10^{-11} \mathrm{~g}\).
This loss in mass is so small that it is negligible. Hence, no detectable change in mass occurs in ordinary chemical reactions.
But in nuclear fission and fusion reactions, the change in mass is released as. nuclear energy.

WBBSE Chapter 3 Stoichiometric Equations Weight Versus Weight Calculations
In stoichiometry, if we have a balanced chemical equation and some measured values then we can use this information to calculate whatever is unknown.
Simple problems based upon stoichiometric equations are of three types:
- Problems on the mass-mass relationship.
- Problems with mass-volume relationship and
- Problems related to vapor density.
Let us illustrate these three types of problems one by one through various examples.
Problems on mass-mass relationship: If the mass of the reactant is given then the mass of the product can be easily calculated and vice-versa is also true.
Wbbse Class 10 Physical Science Notes
To do so, we are to follow three steps: (1) First, we are to convert from mass (in grams) to no. of mol using the formula “no of mol \((n)=\frac{\text { mass given }}{\text { molar mass }}\)
Follow the examples: No. of mol in 48 g of CH4 (C = 12, H = 1),
⇒ \(n=\frac{48}{12+1 \times 4}=3\);
No. of mol in 18g of H2O is \(n=\frac{18}{1 \times 2+16}=1\) and so on.
(2) Then from the balanced equation tries to bring out the relation among known and unknown chemicals in terms of no. of mol.
Because chemical equation gives us a molar relationship, not a mass relationship.
Finally, convert back from no. of mol to grams (mass) using the formula Required mass = (no. of mol) x (molar mass).
For example, mass of 2 mol of H2SO4 = 2 x (1 x 2 + 32 + 16 x 4) = 2 x 98 = 196 g.
Per centage composition of an element in a compound (by weight) :
= \(\frac{\text { mass of that element in the compound }}{\text { total mass of compound }}\) x 100
Example:
Wbbse Class 10 Physical Science Solutions
Question 1. If 28 g of N2 reacts with an excess of H2 then how many grams of NH3 will be produced?
Answer:

Question 2. How many grams of KCIO3 would be required to be heated to produce 48g of O2? (K = 39, Cl = 35-5, O = 16)
Answer:
Wbbse Class 10 Physical Science Solutions

Question 3. If 131 g of Zn reacts with dil. H2SO4, then find the amount of H2gas liberated in the reaction. (Zn = 65-5, H = 1, O = 16)
Answer:

Question 4. How many grams of CaCO3 will be required to produce 2-2 g of CO2 by the action of dil. HCI?
Answer:  Question 5. Due to the thermal dissociation of 10 g of limestone (CaC03), how many grams of quick lime (CaO) and C02 are formed? Also, find the percentage composition of Ca in the given limestone.
Question 5. Due to the thermal dissociation of 10 g of limestone (CaC03), how many grams of quick lime (CaO) and C02 are formed? Also, find the percentage composition of Ca in the given limestone.
Answer: 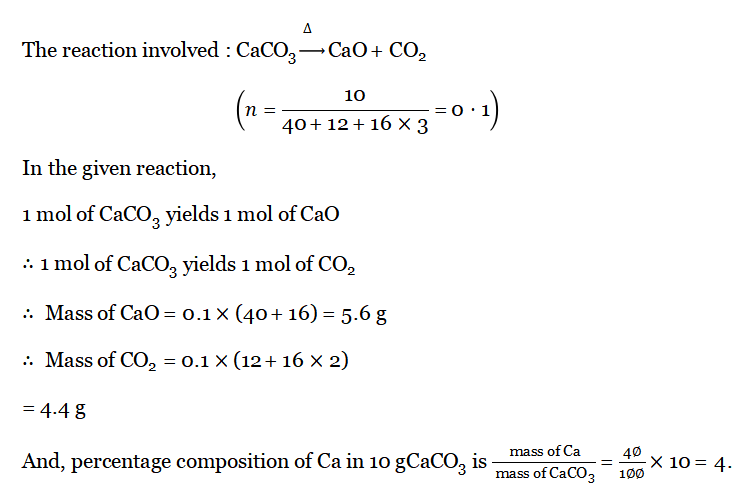 Wbbse Class 10 Physical Science Solutions
Wbbse Class 10 Physical Science Solutions
Question 6. If 48 g of methane is burnt in excess air, find how many grams of C02 will be produced. Also, find the no. of molecules present in that amount of C02
Answer: 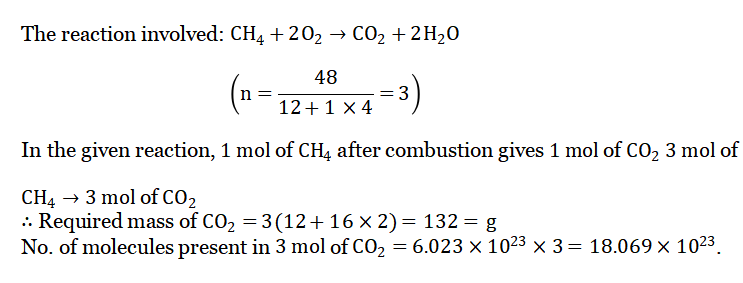
Question 7. To obtain 85 g of NH3, how much amount of NH4CI is to make a reaction with an excess amount of CaO?
Answer:
Wbbse Class 10 Physical Science Solutions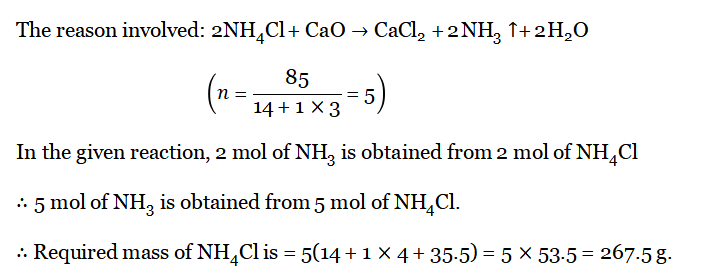
Question 8. How many grams of iron are needed to get 1 mol of hydrogen by running steam on red hot iron? (Fe = 56)
Answer:

Wbbse Class 10 Physical Science Solutions
Problems on mass-volume relationship: If the mass of one reactant is known then the volume of a gaseous product can be calculated or if the volume of a gaseous chemical is known then the mass can be calculated, under similar conditions of temperature and pressure.
In general, this condition would be STP. This is applicable only to gases, not solids or liquids. Like mass
The mass relationship here also we are to follow three steps:
1. Convert volume (at STP) into no. of mol using the formula “no. of mol \((n)=\frac{\text { volume given (at STP) }}{\text { molar volume (at STP) }}=\frac{\text { Volume given }}{22.4 \mathrm{~L}}\)
For example no. of mol of 11-2 L of H2O vapor (at STP) \(n=\frac{11 \cdot 2}{22 \cdot 4}=0 \cdot 5\)
2. Then, from the balanced equation bring out the relation between known and unknown chemicals,
3. Finally, convert back from no. of mol to volume in liters at STP using the formula…
For example Volume of 2 mol of C02 at STP = (no. of mol) (molar volume at STP) = 2 x 22-4 L = 44.8 L.
WBBSE Chapter 3 Stoichiometric Equations Simple Numerical Problems
Question 1. When 367-5 g KCI03 is heated, how many liters of 02 gas will be produced at STP
Answer:
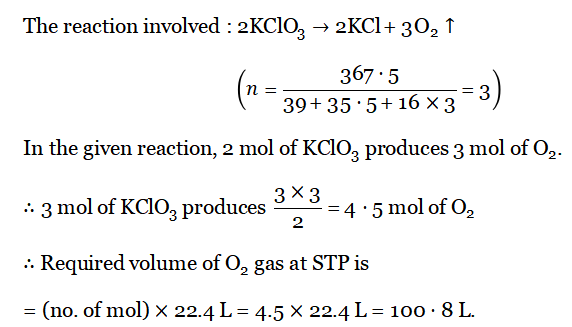
Question 2. On heating some quantity of CaC03, the volume of C02 at STP is measured to be 11-2 L. Find the mass of CaC03.
Answer:
Wbbse Physical Science Class 10

Question 3. Find the volume of CO2 and H2O vapor produced when 64g of CH4 is combusted in presence of excess air.
Answer: 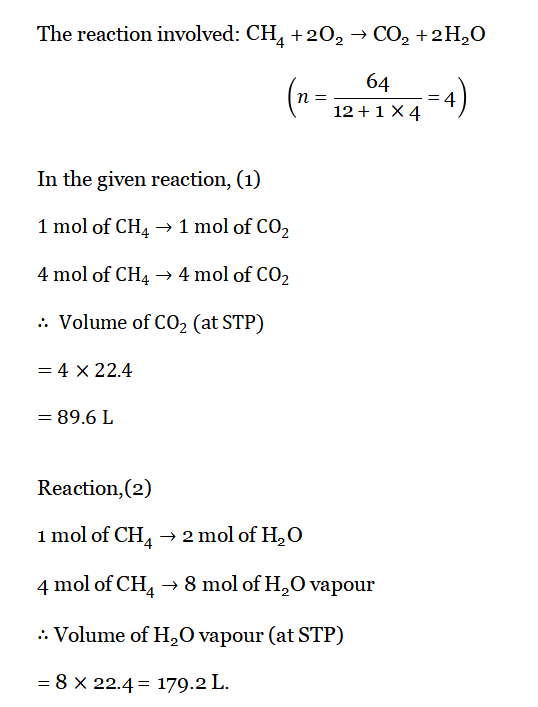
Question 4. Calculate the mass and volume (at STP) Of O2 involved, when 34 g of pure hydrogen peroxide decomposes.
Answer:
Wbbse Physical Science Class 10
Molecular weight and vapor density of gas under a given condition: Gases or vapors have a very low density in comparison to the density of solids and liquids. Usually, the density of a gas is measured in comparison with the density of H2 gas, under similar conditions of T and p.
Definition: The ratio of the density of a gas (or vapor) with the density of an H2 gas under similar conditions of temperature and pressure is called vapor density or relative density of that gas (or vapor).
By definition,
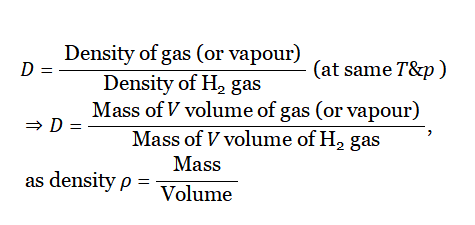
As molar mass for any gas at STP = 22.4 L, so at STP For 1 mol gas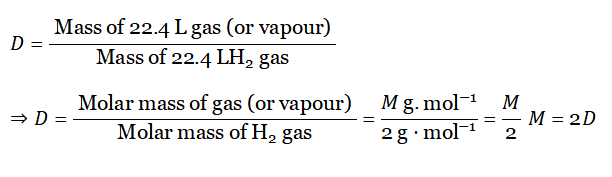
Remember:
- Vapor density is a unitless, dimensionless quantity,
- Vapor density is applicable only for gaseous states of matter,
- M = 2D formula is applicable for an equal volume of both gases at STP.
Question 5. The vapor density of a gas is 11.2. Calculate the volume occupied by 11.2g of the gas at STP.
Answer:
Wbbse Physical Science Class 10
Since M=2D Since M =2×11.2=22.4g
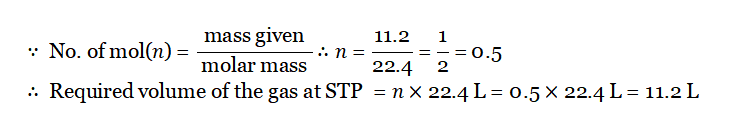
Question 6. Calculate the volume occupied by 40 g Ch4 at STP if its V.D. is 8.
Answer:
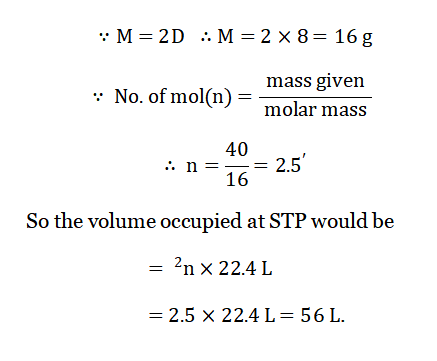
Wbbse Physical Science Class 10
Question 7. Calculate the V.D and Molecular mass of CO2 if 200 ml of the gas at STP weighs 0.40g. (1l of H2 at STP weighs 0.09 g)
Answer: