WBBSE Chapter 7 Atomic Nucleus Radioactivity
Radioactivity Definition: Radioactivity is a nuclear phenomenon of spontaneous emission of α or β and γ-radiations from the nuclei of heavier atoms with atomic number more than 82 (i.e., after 82Pb in the periodic table in order to attain a stable state from an unstable state.)
- In 1896, French scientist Henry Becquerel, while conducting experiments with newly discovered.
- X-rays to investigate how uranium salts are affected by this light and quite accidentally.
- He discovered that the uranium salts spontaneously emit highly penetrating radiations (similar to X-rays) from within themselves.
- The radiations can be registered on a photographic plate. Shortly afterward, Madam Curie and her husband Pierre Curie found that ‘pitchblende’ (an ore of uranium) emits a similar type of radiation.
- They named this phenomenon natural radioactivity and commented that it is a property of the nucleus.
- The phenomenon is ‘spontaneous’ and any physical or chemical condition cannot change the emission of radiation.
- The process of such radiation is known as radioactive decay or disintegration. A nucleus can undergo radioactive -decay through the emission of α or β and γ rays.
- Examples of some radioactive elements are uranium, radium, polonium, thorium, actinium, etc.
Read And Learn Also WBBSE Notes For Class 10 Physical Science And Environment
WBBSE Chapter 7 Atomic Nucleus Nature α,β And γ-Rays
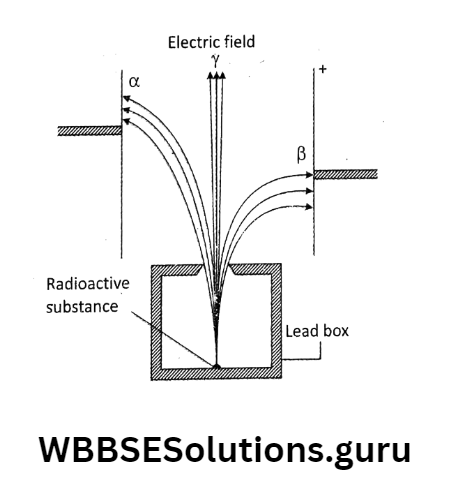
Rutherford and his co-workers discovered that the radioactive rays are a combination of α, β, and γ-radiations.
Comparison of a, p, and y-radiations with reference to some highlighting properties:

How to know the charge of radioactive radiation? Radioactive radiations are affected by an electric field. It is observed that α-particles bent slightly towards the -ve plate.
So they are positively charged heavier particles, β-particles bent more towards -i-ve plate.
So they are negatively charged lighter particles, and γ-radiations remain undeflected so they are uncharged.
Effect on atomic number and mass number due to α, β, and γ-emi-ssion. In any nuclear reaction, total atomic no. and total mass no. always remain the same.
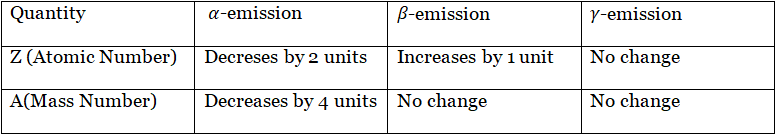
1. Due to the emission of an a-particle, the atomic number decreases by 2 units and the mass number decreases by 4 units. That is,
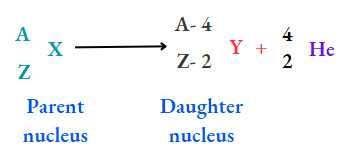
For example,

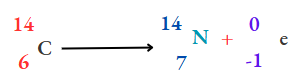
2. Due to the emission of a (3-particle, the atomic number increases by 1 unit, but the mass number remains the same. That is,
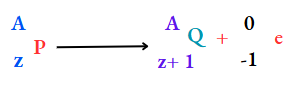
For example,
3. Due to y-radiation, no change in mass and charge occurs.
Note:
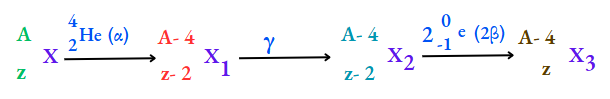
The S.l. unit of radioactivity is Becquerel (Bq). lBq = one disintegration per second or one event per second.
Bigger units: 1 megabecqural or MBq = 106 Bq and 1 gigabecqueral or GBq = 109 Bq The common unit for measuring the activity of radioactive substances is curie.
1 curie = 3.70 x 1010 disintegration per second or 3.70 x1010 event per second.
Smaller units: 1 milli-curie = 10-3 curie and 1 micro-curie = 10-6curie. A unit Rutherford is equal to 106 disintegrations per second.
Smaller units: 1 milli-rutherford = 103 disintegrations per second and 1 micro-rutherford = 106 disintegrations per second.
Note: Radioactivity is considered a nuclear phenomenon (not a chemical phenomenon).
Inside the nucleus of an atom, the repulsive electrostatic force between +vely charged protons (ps) tends to push them away from each other.
For lighter elements whose Z V 20(like \(\left.{ }_2^4 \mathrm{He},{ }_8^{16} \mathrm{O},{ }_7^{14} \mathrm{~N},{ }_{11}^{23} \mathrm{Na},{ }_{12}^{24} \mathrm{Mg},{ }_{20}^{40} \mathrm{Ca}\right)\)
(like for which these nucleons are held together by a strong force (other than electrostatic force) called nuclear force.
This nuclear force cancels out the electrostatic force of repulsion between Ps and keeps the nucleus stable.
The nuclear force is charge-independent, stronger than electrostatic force (~ 100 times), and has a very very short range.
But when the no. of ns exceeds the no. of ps for atoms with \(\mathrm{Z}>20 \text { (like }{ }_{26}^{56} \mathrm{Fe},{ }_{47}^{108} \mathrm{Ag} \text { etc.) }\) for which the ratio → 1.
Then the strong nuclear force cannot hold the ps and ns together and the nucleus becomes unstable.
To attain stability, the unstable nuclei fling out different particles or some energy. This is the reason why some elements are radioactive.
It has been observed that the rate of decay of the radioactive elements remains unaffected:
By any physical change, such as a change in pressure, or temperature,
By any chemical change, such as excessive heating, cooling, oxidation, the action of strong electric and magnetic fields, etc.
3. How β-particles (fast-moving\({ }_{-1}^0 \text { es }\)) are emitted from the nucleus?
β particles are emitted from the nuclei which have too many ns and ps. In order to, maintain the conservation of electric charge, one of the ns Is transformed into ps and vice versa.
This is the reason for (β-decay from the nucleus. In the case of [β-decay, an n is changed into a p and an electron, accompanied by a charge less, massless particle antineutrino.
Although particles and cathode rays both are fast-moving electrons, they differ from one another.
Because; [β-particles are emitted from the nuclei of radioactive elements, whereas cathode rays originate from the orbital electrons.
4. After the emission of α or (γ-particles, the nuclei of radioactive elements acquire a state of excitation where they possess excess energy.
The excess energy is released in the form of electromagnetic radiation, known as the y-radiation so that the excited nuclei come to their ground state.

The y-radiation can be represented as follows:
Although X-rays and y-rays are similar types of electromagnetic radiation, they differ from one another in their origin.
X-rays are emitted due to the transition of electrons in their inner orbits, whereas y-radiations are emitted from the excited nuclei to come to their ground state.
Uses of radioactivity: Radioactivity is used in
(1) Medical field: In the treatment of thyroid, radioactive iodine\(\left({ }_{53}^{131} \mathrm{l}\right)\); in the treatment of blood cancer or leucemia or to detect brain tumor, radioactive phosphorus \(\left({ }_{15}^{32} \mathrm{P}\right)\) in the treatment of malignant cancer, radioactive cobalt \(\left({ }_{27}^{60} \mathrm{Co}\right)\); in the detection of blood circulation in heart, radioactive sodium \(\left({ }_{11}^{24} \mathrm{Co}\right)\) is used.
(2) To determine the age of dead microorganisms (plants, animals), radiocarbon \(\left({ }_{6}^{14} \mathrm{C}\right)\)
(3) The age of older rocks (dating) can be calculated using 238U, 40K radioisotopes.
As rocks often contain traces of uranium, which eventually decays to lead).
(4) In agriculture, radioactive samples are used as tracers and also used in improving food production, improvement of soil fertility, and others.
WBBSE Chapter 7 Atomic Nucleus Mass Defect, Binding Energy, And Nuclear Fission
In the theory of relativity, Albert Einstein explained the equivalence of mass and energy by the equation: E = mc².
According to this law, when an amount of mass ‘m’-disappears, an equivalent amount of energy ‘E’ is released from the system to the surroundings.
Again, if the energy of a system is increased by the amount ‘E’ its mass will be increased by an amount \(m=\frac{E}{c^2}.\)
In the case of high-energy reactions such as nuclear fission and fusion, mass is converted into energy.
In general, the mass of a nucleus is slightly lower than the total mass of the nucleons (protons + neutrons) in the nucleus.
This difference in mass is termed the mass defect (Δm). Some mass disappears. during the formation of the nucleus (exothermic).
Binding energy is the amount of energy released when the nucleons bind together to form the nucleus of an atom.
Suppose that a nucleus has a rest massp\({ }_z^A X\).
If mp and mn are the mass of a proton and a neutron respectively, then the mass defect, \(\Delta m=\left[\mathrm{Z} \cdot m_p+(\mathrm{A}-\mathrm{Z}) m_n\right]-\mathrm{M}\)
Then according to Einstein’s mass-energy equivalence formula, the binding energy will be \(=\Delta m \cdot c^2=\left\{\left[Z \cdot m_p+\right.\right.\left.\left.(\mathrm{A}-\mathrm{Z}) m_n\right]-\mathrm{M}\right\} c^2\)
Nuclear fission: The process of splitting up the nucleus of a heavy atom after interacting with the projectile (mainly thermal neutron having energy ~ 0.025 eV) into two or lighter nuclei of comparable size with the release of energy is known as nuclear fission.
A typical example of a nuclear fission reaction is a single neutron \(\left({ }_0^1 n\right)\) in a uranium nucleus \(\left({ }_{92}^{235} \mathrm{U}\right)\) produces three neutrons accompanied by two comparatively lighter nuclei of barium and krypton.
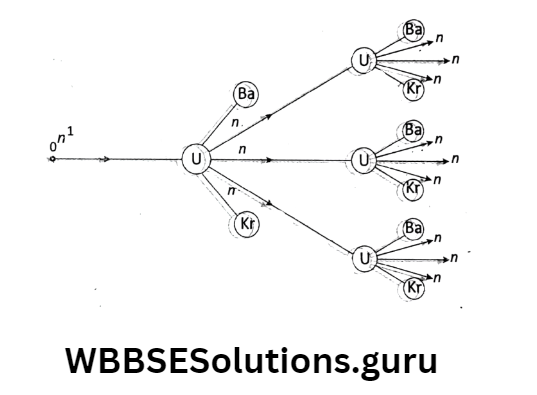
The three neutrons produced in the reaction disintegrate three more \(\left({ }_{92}^{235} \mathrm{U}\right)\) nuclei. Then, nine neutrons are produced, which in turn can produce fission of nine more\(\left({ }_{92}^{235} \mathrm{U}\right)\) nuclei and so on.
Thus a chain reaction would start and within a few milliseconds, a tremendous amount of energy would be liberated. The daughter nuclei are also radioactive and they further emit [β-rays, and γ-rays.
Calculation of energy released: For nuclear fission reaction, all the masses are taken in a.m.u. or u (where 1 u= 1.66 x 10-27Kg).
Total mass before reaction = 234.993 u + 1.00 87 u = 236.0022 u
Total mass after reaction = 140.8834 u + 91.9020 u + 3 x 1.0087 u = 235.8115 u
∴ Difference in mass (i.e., mass defect) = 236.0022 u – 235.8115 u = 0.1907 u
According to the equation E = me2, 1 u mass is equivalent to 932-6 MeV energy.
Thus, one nucleus of 235U releases the energy = 0-1907 x 932-6 MeV = 177-85 MeV.
Can you imagine the fission of only 50 g of 235U? 3-55 x 1012 J (very large amount) of energy is released by which one 100 W bulb could run for about 1125 years
Atom bomb: The principle of nuclear fission (using U-235 or Pu-239) is used in the construction of an atom bomb.
In it, the fission reaction takes place in an uncontrolled manner so that a single fission causes more than fission and an enormous amount of energy (as I energy) is liberated within a few milliseconds. The how an atomic bomb is made.
During the final stage of World War II, two a-bombs were dropped on the Japanese cities Hiroshima (on Aug 6, 1945, at 8:11 am, named “Li Boy’) and Nagasaki (on Aug 9, 1945, at 11: 02 am, explosion and it’s named ‘Fat Man’).
The two bombings killed at least 129000 people, and destroyed a lot like burning effects, radiation sickness, genetic defects, loss of fertility of lands, and many others.

Nuclear reactors as peaceful use of nuclear energy: A nuclear reactor is a device in which a controlled nuclear chain reaction can be initiated to produce energy.
In a controlled nuclear reaction, a single fission causes one fission, and energy is liberated at a constant rate.
This energy is used at nuclear power plants for the generation of electricity. Today, about 450 or more nuclear reactors are used in about 30 nations around the world.
Physics Class 10 Wbbse
Nuclear accidents :
1. The Chornobyl accident was the worst nuclear power plant accident in history. It happened on 26 April 1986 in Ukraine (part of the former Soviet Union).
Operating the plant at very low power, the reactors became highly unstable, and hot (about 2000°C) radioactive fuel substances came out as a steam explosion.
It lifted the cover off the top of the reactor. A large area was contaminated within 36 hours of the accident.
The Fukushima accident (located in the northern part of Japan) occurred on 11 March 2011 following a massive earthquake (recorded magnitude 9-0) and tsunami (about 15 m high).
The plant’s emergency power generators in the basement were flooded, causing the leakage of radioactive substances into the environment.
The fuel rods were also melted with the leakage of deadly radiation. It is the worst nuclear disaster since the 1986 Chornobyl accident along with the release of a large amount of energy is called nuclear fusion.
The process of the formation of a heavy nucleus by the combination of two or lighter nuclei \(\left({ }_1^1 H,{ }_1^2 H,{ }_1^3 \mathrm{H},{ }_3^6\right. \text { Li, etc.) }\)
A typical example of a fusion reaction is:
⇒ \(4{ }_1^1 \mathrm{H} \longrightarrow{ }_2^4 \mathrm{He}+2{ }_{+1}^0 e+\text { Energy or, }{ }_1^2 \mathrm{H}+{ }_1^3 \mathrm{H} \longrightarrow{ }_2^4 \mathrm{He}+{ }_0^1 \mathrm{n}+\text { Energy }\)
The difference in mass between the parent nuclei and the daughter nuclei is released in the form of energy (approximately 17-6 MeV energy perfusion).
Physics Class 10 Wbbse
The nuclear fusion reactions take place at a very high temperature (about 107°C). Due to this fact, nuclear fusion is also called thermonuclear reaction.
Such a high temperature can result only from a nuclear fission reaction. But, once the nuclear fusion reaction starts, the energy release is so high enough to maintain a high temperature to fuse more nuclei and the reaction continues.
That’s why, we say nuclear fission initiates nuclear fusion. Mathematically, it can be proved that in fusion much more amount of energy is released than in fission reaction per unit mass of fuel.
Nuclear fusion is not a chain reaction.
Physics Class 10 Wbbse
Nuclear fusion takes place in the interior of the sun and other stars. Sun and other stars are composed of about 90% hydrogen and helium gas. Only 10% is other elements…
In a hydrogen bomb, the synthesis of a heavier atom from hydrogen results in the release of an enormous amount of energy.
