WBBSE Chapter 8 Inorganic Chemistry In The Laboratory And In Industry Laboratory Preparation Of Ammonia(NH3)
Principle: Ammonium salt + Alkali → Salt + Ammonia Gas+ H2O.
When ammonium salts are heated with less volatile strong alkalis, then comparatively more volatile ammonia gas is produced. For example:
⇒ \(\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4(s)+\mathrm{CaO}(s) \stackrel{\Delta}{\longrightarrow} \mathrm{CaSO}_4(s)+2 \mathrm{NH}_3(g)+\mathrm{H}_2 \mathrm{O}(I)\)
⇒ \(\mathrm{NH}_4 \mathrm{Cl}(s)+\mathrm{NaOH}(\text { aq. }) \stackrel{\Delta}{\longrightarrow} \mathrm{NaCl}(\mathrm{s})+\mathrm{NH}_3(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(I)\)
⇒ \(\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4(s)+2 \mathrm{KOH}(\text { aq. }) \stackrel{\Delta}{\longrightarrow} \mathrm{K}_2 \mathrm{SO}_4(s)+2 \mathrm{NH}_3(g)+2 \mathrm{H}_2 \mathrm{O}(I)\)
Caustic alkalis like NaOH/KOH are not used because they are deliquescent i.e. from an atmosphere they absorb moisture and dissolve.
In the laboratory, slaked lime [Ca (OH)2] is used as an alkali.

Chemicals required: Dry ammonium chloride (NH4CI) and dry Ca (OH)2in 1: 3 mass ratio (Both are finely ground).
Condition:
- Direct contact of the reactants and
- Application of heat.
Chemical equation: \(2 \mathrm{NH}_4 \mathrm{Cl}(s)+\mathrm{Ca}\begin{aligned}
& (\mathrm{OH})_2(s)-\stackrel{\Delta}{\longrightarrow} \mathrm{CaCl}_2(s)+2 \mathrm{NH}_3(g)+ \\& 2 \mathrm{H}_2 \mathrm{O}(g)\end{aligned}\)
Laboratory arrangement: The round bottom flask is kept in a tilting position.
Drying agent: Drying is essential because water vapor is one of the products.
Moist NH3(g) is dried by passing it through quick lime (CaO). Ammonia gas is basic in nature. Quicklime is also a base. So they never react to one another.
WBBSE Notes For Class 10 Physical Science And Environment
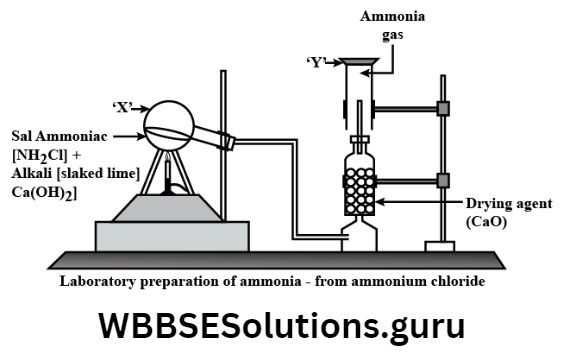
Gas collection: Dry NH3(g) is collected by downward displacement of air in the inverted gas jar. Because-
- NH3(g) is lighter than air and
- NH3 (g) is highly soluble in water. A moist red litmus paper held near the mouth of the gas jar turns blue in the presence of NH3 (g).
Precautions:
The reagents are finely ground in dry condition, otherwise, NH4CI may sublime on heating,
The round bottom flask is kept tilted so that water may not trickle back and crack the hot flask,
In the laboratory process, we should not use ammonium nitrate (NH4NO2).
Because NH4NO2 is explosive in nature, it decomposes on heating forming nitrous oxide (N2O) gas and water vapor.
⇒ \(\mathrm{NH}_4 \mathrm{NO}_3(s) \stackrel{\Delta}{\longrightarrow} \mathrm{N}_2 \mathrm{O}(g)+2 \mathrm{H}_2 \mathrm{O}(g)\)
In drying NH3(g), common drying agents are like a cones. H2SO4, P2 O5, and anhydrous CaCl2 are not used.
Because NH3 reacts with these substances: 2NH3 + H2SO4 →(NH4)2SO4; 6NH3 + P2O5 + 3H2O→ 2 (NH4)3 PO4 (P2O5 is acidic in nature); x NH3 + (anhydrous) CaCI2 → CaCI2 . × NH3 (addition compound)
WBBSE Chapter 8 Inorganic Chemistry In The Laboratory And In Industry Properties Of Ammonia
Physical properties: Ammonia is a colorless gas having a characteristic pungent choking smell.
The pungent smell in the washroom is due to ammonia.
Density: Under standard conditions, the specific density of ammonia is 8-5 whereas the specific density of air is 14-4. This means that NH3 (g) is lighter than air.
Solubility: At ordinary temperature, NH3 (g) is highly soluble in water (in 1 volume of water about O2 volume of NH3 (g) is soluble).
Aqueous ammonia: Ammonia, being very much soluble, is dissolved in water and forms ammonium hydroxide (NH4OH) which is a weak base due to the presence of OH¯ ions.
⇒ \(\mathrm{NH}_3+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{NH}_4 \mathrm{OH} ; \mathrm{NH}_4 \mathrm{OH} \rightleftharpoons \mathrm{NH}_4^{+}+\mathrm{OH}^{-} \text {(Arhenious theory) }\)
This is called aqueous ammonia (or in the aqueous form of ammonia) called ammonium hydroxide. Under high pressure, the volume of NH3 (g) is compressed, and when the temperature is decreased, the gas gets liquified.
By cooling NH3(g) to a temperature – 33-4°C under normal pressure, it changes into a colorless liquid called anhydrous or liquid ammonia /moisture-free ammonia.
Liquor ammonia: It is the 35% concentrated/saturated solution of ammonia in water. Its specific gravity is 0.88. That’s why liquor ammonia is treated as a very strong solution of NH3 (g) in water.
Remember: Liquor ammonia is not liquid ammonia. Liquor ammonia contains NH4OH (NH4+and OH¯ions) whereas liquid ammonia contains NH3
Chemical properties:
Reactions due to basic nature: Ammonia is a weak base, so reacting with acids (HCl, H2S04) it forms ammonium salts.
For example:
⇒ \(\mathrm{NH}_3 \text { (aq.) }+\mathrm{HCl} \text { (aq:) } \rightarrow \mathrm{NH}_4 \mathrm{Cl} \text { (aq.) (ammonium chloride salt) }\)
The same reaction happens in the gaseous state also.
⇒ \(\mathrm{NH}_3(g)+\mathrm{HCl}(g) \rightarrow \mathrm{NH}_4 \mathrm{Cl}(s) \text { (dense white fumes) }\)
When a glass rod is dipped in a cone. HCl is brought in contact with NH3 (g), and it produces dense white fumes (fine dust of solid NH4CI).
This is a reaction between two gases that produces a solid compound. This reaction is also used for the detection of ammonia gas.
⇒ \(2 \mathrm{NH}_3 \text { (aq.) }+\mathrm{H}_2 \mathrm{SO}_4 \text { (aq.) } \rightarrow\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4 \text { (ammonium sulphate salt) }\)
NH4OH (aq.) turns red litmus blue and colorless phenolphthalein solution pink.
Reducing property: NH3 is a good reducing agent. This means NH3 reduces i.e. removes O2 from another substance. Example:
When NH3 (g) is passed over red hot copper (II) oxide (black), then NH3 reduces CuO to form metal copper [red).
At the same NH3 gets oxidized in N2 (g). This reaction proves that NH3 contains nitrogen.
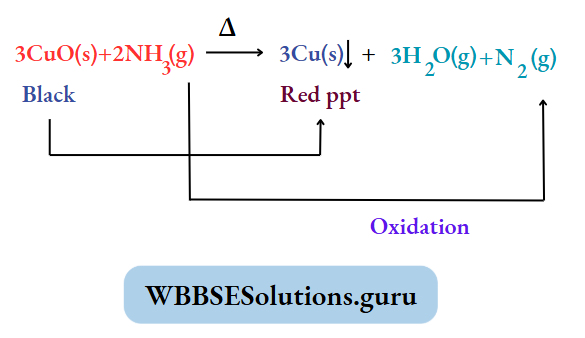
Catalytic oxidation of NH3:
⇒ \(4 \mathrm{NH}_3(g)+5 \mathrm{O}_2(g) \frac{\mathrm{Pt}}{800^{\circ} \mathrm{C}} 4 \mathrm{NO}(g)\)+ 6 \(\mathrm{H}_2 \mathrm{O}(g)+\text { Heat (Exothermic reaction). }\)
NH3 (g) reacts with O2 in the presence of Pt- catalyst heated at 800°C and colorless nitric oxide gas (NO) is produced (oxidation reaction).
⇒ \(4 \mathrm{NH}_3+3 \mathrm{O}_2 \rightarrow 2 \mathrm{~N}_2+6 \mathrm{H}_2 \mathrm{O}(\mathrm{g})\)
The oxidizing tendency is so high that NO (g) further oxidizes into reddish-brown nitrogen dioxide gas (NO2).
⇒ \(\mathrm{NO}(g)+\mathrm{O}_2(g) \rightarrow \mathrm{NO}_2(g) \text { (reddish brown gas) }\)
4. Precipitation of metal hydroxides from aq. solution of metal ions (Fe3+, Al3+) :
Analytical chemistry: Usually, all cations like Na+, K+, Ca2+, Zn2+, Mg2+, Al3+, NH4+ are colourless and all anions like Cl–, Br–, CO32-, NO31-, SO4z-
Are colorless except for some cations: Fe2+ (-ous) → light green, Fe3+ (-ic) → yellowish/brown, Cu2+ (-ous) → blue and some anions MNO41- (permanganate) →pink, Cr2O72- (dichromate) → orange.
That’s why Zn (NO3)2 → colourless, FeSO4 → light green, FeCI3 →yellow, AICI3 → colourless, CuSO4 → blue, …. etc.
Here we will study what happens due to the addition of NH4OH/ NaOH solution into the aq. solution of Fe3+ (ferric), and Al3+ salts—especially to note down the color of the salt and its solution.
⇒ \(\begin{array}{ll}
\mathrm{FeCl}_3 \text { (aq.) }+3 \mathrm{NH}_4 \mathrm{OH}(\text { aq.) } \longrightarrow & \mathrm{Fe}\left(\mathrm{OH}_3\right)(s) \downarrow+3 \mathrm{NH}_4 \mathrm{Cl} \text { (aq.) } \\
\text { yellow } & \text { Brown ppt. } \\
\mathrm{AlCl}_3 \text { (aq.) }+3 \mathrm{NH}_4 \mathrm{OH} \longrightarrow & \mathrm{Al}(\mathrm{OH})_3(s) \downarrow+3 \mathrm{NH}_4 \mathrm{Cl} \text { (aq.) } \\
\text { Colourless } & \text { Gelatinous } \\
\text { white ppt. }
\end{array}\)
Significance of such reactions: Observing the color of metal hydroxide precipitate, we can easily detect /identify the presence of metallic cation (Fe3+, Al3+).
Physical observation: In aq. CuSO4, aq. NH3 is added :
⇒ \(\mathrm{CuSO}_4 \text { (aq.) }+2 \mathrm{NH}_4 \mathrm{OH} \text { (aq.) } \longrightarrow \mathrm{Cu}(\mathrm{OH})_2(\mathrm{~s}) \downarrow+\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4 \text { (aq.) }\)
But this blue ppt. of Cu(OH)2 dissolves in excess NH4OH forming a deep blue soluble com plex salt-tetramine copper (II) sulfate.
The alkaline solution of potassium mercuric iodide (K2Hgl4) is called Nessler’s reagent. It is used to identify/test the presence of ammonia.
Ammonia in contact with Nessler’s reagent produces yellowish/brown ppt. A bottle of liquor ammonia is cooled before opening.
We know that a large quantity of NH3 vapor is kept under high pressure inside the bottle. If the bottle is extremely suddenly opened due to the release of pressure, NH3 vapor may spurt danger to the eyes, out and come in contact with the eyes.
This is very much harmful to the eyes.
Use of liquid ammonia as a coolant: It is used as a refrigerant in ice plants.
Liquid NH3 is capable of absorbing heat as its specific heat capacity is very high and cools another substance.
In the refrigerator, tetra fluoro ethane is used. Why not liquid NH3? Because- NH3 reacts with Cu (refrigerator pipe metal) a high quantity of NH3 is poisonous.
Ammonia gas is non-poisonous. If inhaled, affects the respiratory system and brings tears to the eyes.
NH3 concentration (300 ppm) is immediately dangerous to life. Workers should use personal protective equipment:
In case of accidental leakage of NH3 from cold storage, the first and foremost duty is to wash out the affected body parts with water as NH3(g) is highly soluble in water.
In the factory, after NH3 (g) pipeline leakage inhalation of NH3 can cause severe irritation of the nose and throat, coughing, and shortness of breath/difficulty in breathing resulting in respiratory failure. People fell unconscious and they need to be immediately hospitalized.
WBBSE Chapter 8 Inorganic Chemistry In The Laboratory And In Industry Major Industrial Uses And Industrial Manufacture Of NH3 And Urea
Industrial uses of ammonia: Ammonia is used in the manufacture of
Nitrogenous fertilizers: examples – ammonium sulfate [(NH4)2SO4], ammonium nitrate (NH4NO3), ammonium phosphate [(NH4)3P04], urea [CO (NH2)2], etc.
Nitric acid by Ostwald’s process, sodium carbonate (Na2CO3) by Solvay’s process
Explosives: examples-TNT (tri nitro toluene), RDX, etc.
Polymers: Examples – nylon, rayon, plastics, dyes, etc.
Ammonium chloride is used in dry cells, ammonium carbonate is used as a smelling salt for reviving a fainted person
As a refrigerant in ice-plants
Many pharmaceutical products, and household cleaning products – removing grease/perspiration strains, cleaning tiles/windows, etc.
Industrial manufacture of NH3 (Haber’s process): (For industrial production on a large scale) From the synthesis of dry and pure N2 (g) and H2 (g) in the ratio 1 : 3 by volume under some favorable conditions NH3 (g) is produced.
Favorable conditions:
High temperature: 450 – 500°C
High pressure: 200 – 900 atm
Catalyst: Finely divided Fe
Promoter: Molybdenum (Mo) or Al2O3
Relevant Reaction:
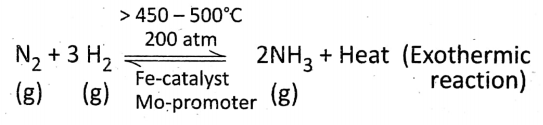

In this reaction, both the reactants and product are gaseous; the reaction is reversible and exothermic; volume decreases in the forward direction.
Collection Of NH3: The gaseous mixture contains produced NH3 along with unrelated N2 and H2 Then by condensation through a cooling chamber, NH3 (g) is liquefied easily as compared
to N2 and H2 molecules and dissolved in water (because NH3 is highly soluble in water).
By recirculating unreacted N2 and H2, an eventual yield of NH3 (~ 98%) can be obtained.
Uses of urea [CO (NH2)2]: Urea is used both as a nitrogenous fertilizer (about 90%) and animal feed.
In the manufacture of urea-formaldehyde resin/plastic used for lamination/fabrication purposes.
In the production of urea stibine, a medicine for leishmaniasis (kala-azar), and barbiturates, used as sleeping pills.
Manufacture of urea: Raw materials are: CaCO3 (indirectly used). \(\mathrm{CaCO}_3 \stackrel{\Delta}{\longrightarrow} \mathrm{CaO}+\mathrm{CO}_2\)
This CO3 is used in urea production, NH3 (directly used through Haber’s process).
Condition: Liquid NH3 and liquid CO2 react at 200°C temperature Under 200 atm pressure.
Chemical reaction:

WBBSE Chapter 8 Inorganic Chemistry In The Laboratory And In Industry Laboratory Preparation Of Hydrogen Sulphide(H2S)
Principle: A strong acid can substitute the weak acid part from its salt. Example- (where H from strong acid HCI substitutes Na of Na2S)
Chemicals required : A few pieces of solid ferrous sulfide (FeS) [dark brown in color] and
H2SO4(or dil. HCI).
Condition: Direct contact of FeS and dil. H2SO4 at normal temperature.
Chemical equation: FeS (s)+ H2SO4(aq.) →FeSO4+ H2S (g)
(where H2SO4→strong acid, H2S→ weak acid, FeS→ salt of weak acid)
Laboratory arrangement: H2S (g) is collected by the upward displacement of air, as it is heavier than air.
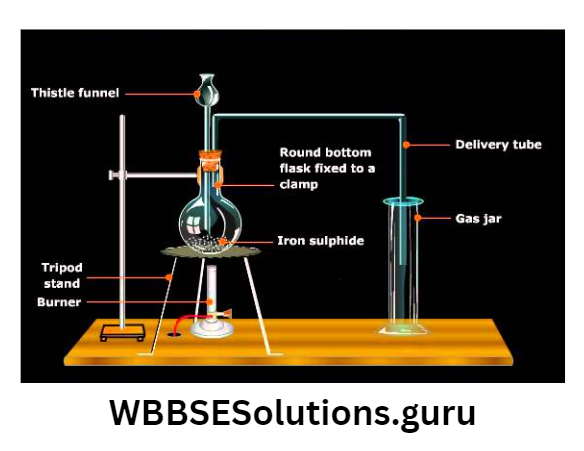
How Laboratory make H2S(g) free from water vapor? this is done by passing H2S (g) through acidic Phosphorous pentoxide (P2Os) (because H2S is acidic and reducing in nature).
Why drying agents like
conc. H2SO4 Quicklime(CaO) or Anhydrous CaCl4are not used? Conc. H2SO4 is a strong oxidises H2S to sulfur(s)
⇒ \(\mathrm{H}_2 \mathrm{SO}_4 \text { (aq.) }+\mathrm{H}_2 \mathrm{~S}(g) \longrightarrow 2 \mathrm{H}_2 \mathrm{O}(I)+\mathrm{SO}_2(g)+\mathrm{S}(s)\)
CaO is a base. \(\mathrm{CaO}(s)+\mathrm{H}_2 \mathrm{~S}(g) \longrightarrow \mathrm{CaS}(s)+\mathrm{H}_2 \mathrm{O}\)
(anhydrous s) \(\mathrm{CaCl}_2+\mathrm{H}_2 \mathrm{~S}(g) \rightarrow \mathrm{CaS}(s)+2 \mathrm{HCl}(g)\)
In the laboratory preparation of H2S(g) from FeS (s)why conc. H2SO4 conc. HNO3 conc. H2SO4 is not taken?

Reaction: FeS + H2SO4 → FeSO4 + HS
A, B, and C are respectively bottom, middle, and top chambers or globes of Kipp’s apparatus. Chamber C connects chamber A passing through middle chamber B. A tap is fitted to the middle chamber B.
Take solid FeS in B. Add dil. H2SO4 through C, until acid touches FeS in B. At once, the reaction starts and H2S (g) is produced. By opening the tap, H2S (g) can be collected.
When the tap remains closed (air-tight), the high pressure of gas in B pushes H2S04 down to bottom chamber A and up into the top chamber C.
This separates FeS and H2SO4. Immediately, further production of gas is stopped.
As per need, when a tap is opened, pressure in the middle chamber decreases, which allows H2SO4 to enter into B from A making contact with FeS. Again H2S (g) starts producing.
WBBSE Chapter 8 Inorganic Chemistry In The Laboratory And In Industry Properties Of Hydrogen Sulphide
Physical properties: Foul odor of rotten egg, colorless, poisonous gas.
G Density: Heavier than air. H2S has a vapor density of 1-2, it is large as compared to air which is 1 Solubility Moderately soluble in cold water. But insoluble in hot water.
Chemical properties:
Acidic property: Aq. solution of H2S (g) behaves like a mild acid. It is a di-basic acid.
⇒ \(\mathrm{H}_2 \mathrm{~S}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{HS}^{-} \text {i.e. } \mathrm{H}_2 \mathrm{~S} \text { (aq.) } \rightleftharpoons \mathrm{H}^{+}+\mathrm{HS}^{-}\)(partial ionization)
⇒ \(\mathrm{HS}^{-}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{S}^{2-}+\mathrm{H}_3 \mathrm{O}^{+} \text {i.e. } \mathrm{H}_2 \mathrm{~S} \text { (aq.) } \rightleftharpoons 2 \mathrm{H}^{+}+\mathrm{S}^{2-}\)(complete ionization)
Reaction with alkali [ex. NaOH (aq.)]: Since aq. H2S behaves as a di-basic acid, it reacts with NaOH solution to form both acid salt and normal salt.
⇒ \(\begin{array}{ll}
\mathrm{NaOH}+\mathrm{H}_2 \mathrm{~S} \longrightarrow & \mathrm{NaHS}+\mathrm{H}_2 \mathrm{O} \text { (partial ionization) } \\
& \text { acid salt (sodium hydrogen sulfide) } \\
\mathrm{NaHS}+\mathrm{NaOH} \longrightarrow & \mathrm{Na}_2 \mathrm{~S}+\mathrm{H}_2 \mathrm{O} \text { (complete ionization) } \\
\text { normal salt (sodium sulfide) }
\end{array}\)
Combustibility of H2S (g): H2S (g) burns in air (O2) with blue flame but does not support in burning.
In the excessive supply of O2, sulfur dioxide gas (another toxic gas) is produced, and in low O2-level, sulfur deposits.
(excessive supply of O2) 2H2S + 3O2 2H2O + 2SO2 (low supply of O2) 2H2S + O2→2H2O+ 2S
As a strong reducing agent (reaction with acidified potassium dichromate solution): H2S (g) is passed through acidified K2Cr2O7 solution (orange).
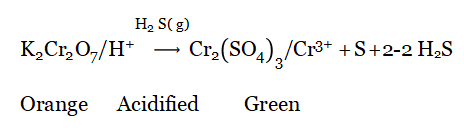
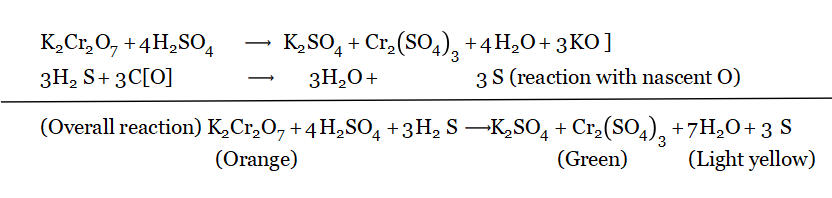
Result: Orange Coloured Solution Solution is Converted Into a Green Colour
This Colour Change occurs only because of the reducing property of H2S.
(4) Precipitation of mental sulfides (Cus, Pbs, Ag2S- black) From Aqueous solution of copper sulfate CuSO4 (blue), lead nitrate pb (NO3)2 (Colourless): H2S (g) is passed through the Queous solutions separately in each case, Black ppt. of mental sulfide is obtained.
⇒ \(\begin{array}{ll}
\mathrm{CuSO}_4 \text { (aq.) }+\mathrm{H}_2 \mathrm{~S}(g) \longrightarrow & \mathrm{CuS}(s) \downarrow+\mathrm{H}_2 \mathrm{SO}_4 \text { (aq.) } \\
\text { (Blue) } & \text { (Black) } \\
\mathrm{Pb}\left(\mathrm{NO}_3\right)_2 \text { (aq.) }+\mathrm{H}_2 \mathrm{~S}(g) \longrightarrow & \mathrm{PbS}(s) \downarrow+2 \mathrm{HNO}_3 \text { (aq.) } \\
\text { (Colourless) } & \text { (Black) } \\
2 \mathrm{AgNO}_3 \text { (aq.) }+\mathrm{H}_2 \mathrm{~S}(g) \longrightarrow & \mathrm{Ag}_2 \mathrm{~S}(s) \downarrow+2 \mathrm{HNO}_3 \text { (aq.) } \\
\text { (White) } & \text { (Black) }
\end{array}\)
Identification test for H2S: Dip a filter paper into lead acetate solution, which in contact with H2S (g) immediately turns black due to the formation of insoluble black lead (II) sulfide.
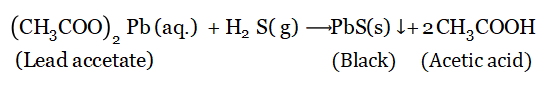
This method is important to determine the presence and amount of H2S (g).
Reaction with alkaline sodium nitroprusside solution: When H2S {g) is passed through freshly prepared alkaline sodium nitroprusside solution (colorless), the solution becomes violet.
Toxicity of H2S: It is a highly toxic gas.
Rapidly affects the central nervous system and respiratory system. Inhaling high concentrations (over 500 -1000 ppm) can cause instant death.
There is no proven antidote for H2S poisoning. Wear a nose mask and avoid inhaling H2S.
WBBSE Chapter 8 Inorganic Chemistry In The Laboratory And In Industry Laboratory Preparation And Major Uses Of Nitrogen (N2)
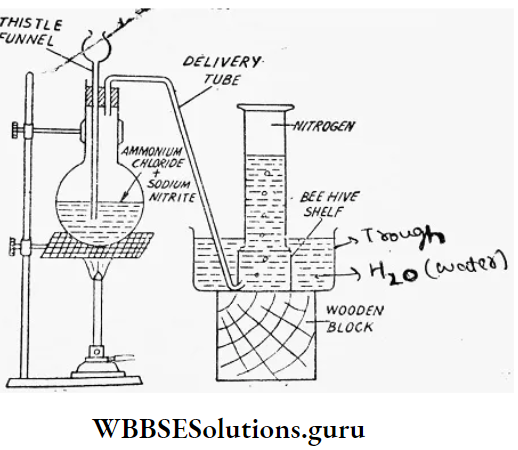
Laboratory preparation: Chemical required: A concentrated aqueous solution of ammonium chloride (NH4CI) and sodium nitrite (NaNO2) in 1:1 molar proportion.
Condition: In the laboratory N2(g) is prepared by (gently) heating the mixture of NH4CI and NaNO2.
Chemical reaction: The reactants initially undergo double decomposition to form ammonium nitrite (NH4NO2) and sodium chloride (NaCI). NH4NO2 (aq.) + NaCI (aq.)
⇒ \(\mathrm{NH}_4 \mathrm{Cl} \text { (aq.) }+\mathrm{NaNO}_2 \text { (aq.) } \stackrel{\Delta}{\longrightarrow}\)
⇒ \(\mathrm{NH}_4 \mathrm{NO}_2 \text { (aq.) }+\mathrm{NaCl} \text { (aq.) }\)
Thereafter the NH4NO2 formed here decomposes to produce N2 (g).
⇒ \(\mathrm{NH}_4 \mathrm{NO}_2 \text { (aq.) } \stackrel{\Delta}{\longrightarrow} \mathrm{N}_2(g)+2 \mathrm{H}_2 \mathrm{O}(g)\)
Gas collection: N2(g) is collected by the downward displacement of water.
Drying of laboratory-prepared N2 (g): By passing through
Cone. H2S04, existing moisture can be removed
Cone. NaOH/KOH, Cl2 (g) can be removed
Finally passing over red hot Cu, oxides of nitrogen are removed (reduced) to form fresh N2 (g).
Main uses: N2(g) is used in the industrial manufacture of NH3 (by Haber’s process.
NH3 is an important starting material in the production of HNO3 (by Ostwald’s process), nitrogenous fertilizers such as (NH4)2SO4, NH4NO2, (NH4)3PO4, etc, explosives, and pharmaceutical products.
Being an inert gas creates an unreactive atmosphere inside an electric bulb, and thermometer to prevent oxidation.
In many industrial pros, N9 boils at —196 C. So, liquid N2 is used cesses it is used to create an unreactive atmo- 2 ………. 2 to cool down the exothermic reaction, sphere, as N2 is cheaper than He/Ar.
Packets of chips/pop-corn/ other food packets are made puffy using N2 (g) so that oxidation and growth of bacteria are stopped.
The food products also remain fresh. That’s why N2 is very important in food packaging.
Liquid N2 has many real-life applications such as the preservation of biological specimens example: blood, cornea, eye, tissue samples, etc.
WBBSE Chapter 8 Inorganic Chemistry In The Laboratory And In Industry Properties Of N2
Physical properties: N2a colorless, odorless, and tasteless gas.
Density: Slightly less dense than air (vapor density of N2 and air are respectively 14 and 14-4).
Solubility: N2 (g) is sparingly soluble in water (1L of water dissolves 22 mL of N2 at 0°C). 0 N2 (g) freezes at – 210-1°C.
At high pressure, N2 (g) can be liquified. N2 boils at – 196°C.
Chemical properties: The molecular structure of nitrogen is ![]() However each N2 molecule is very difficult to break and that’s why N2 molecules have a very strong triple bond shared between two N atoms which behave like an inert/unreactive gas at normal temperature.
However each N2 molecule is very difficult to break and that’s why N2 molecules have a very strong triple bond shared between two N atoms which behave like an inert/unreactive gas at normal temperature.
But at extremely high temperatures N2 can react with other metals/non-metals. For example—
Reaction with hydrogen: Mixture of pure and dry N2 (g) and H, (g) in the volume ratio 1 : 3 when passed over hot (450 – 500°C) finely divided Fe-catalyst and Mo-promoter under high pressure (> 200 atm) then NH3 (g) is produced through an exothermic reaction.
This is the principle of industrial production of NH3 by Haber’s process.
⇒ \(\mathrm{N}_2+3 \mathrm{H}_2 \underset{\substack{450-500^{\circ} \mathrm{C} \\ \text { above } 200 \mathrm{~atm}}}{\stackrel{\mathrm{Fe} \text { and } \mathrm{Mo}}{\rightleftharpoons}} 2 \mathrm{NH}_3+\text { Heat }\)
Reaction with magnesium: N2 (g) puts off a burning candle as it is neither combustible nor a supporter of combustion (inert gas).
But a burning element can react with N2 exactly what happens in the case of a burning magnesium ribbon placed in an Infilled gas jar.
Mg-ribbon burns and forms a yellowish powder called magnesium nitride (Mg3N2).
Chemical reaction: \(3 \mathrm{Mg}(s)+\mathrm{N}_2(g) \stackrel{\text { Heat }}{\longrightarrow} \mathrm{Mg}_3 \mathrm{~N}_2(s)\)
Reaction with calcium carbide: Formation of nitrolim: When N2(g) is passed over hot (~ 1100°C) calcium carbide dust (CaC2), a brownish grey solid mixture is produced which contains calcium cyanamide (CaNCN) and carbon (C)…
noo°c nitro slim Commercially, this solid mixture (CaNCN + C) is called nitrolim- an inorganic compound used as a chemical fertilizer.
Nitrogen fixation: Nitrogen is present in abundance (~ 78%) in the atmosphere. Plants and animals cannot take nitrogen directly from the atmosphere.
So, anyhow N2 needs to be converted into its usable forms (usually nitrogenous compounds). This process of conversion of atmospheric N2 into useable forms is referred to as “nitrogen fixation.”
N2 combines with O2 in the presence of lightning at a temperature of 3000 – 5000°C to form nitric oxide (NO), and NO further oxides into NO2 (nitrogen dioxide).
⇒ \(\mathrm{N}_2(g)+\mathrm{O}_2(g) \stackrel{\text { lightning }}{\longrightarrow} 2 \mathrm{NO}(g) ; 2 \mathrm{NO}(g)+\mathrm{O}_2(g) \stackrel{\text { lightning }}{\longrightarrow} 2 \mathrm{NO}_2(g)\)
During rainfall, NO2 (g) reacts with water and produces both aq. nitric acid (HN03) and un stable aq. nitrous acid (HNO2) and fall down to the soil.
⇒ \(2 \mathrm{NO}_2(g)+\mathrm{H}_2 \mathrm{O}(l) \rightarrow \mathrm{HNO}_3 \text { (aq.) }+\mathrm{HNO}_3 \text { (aq.) }\)
Alkaline substances present in soil react with aq. HNO3 forms nitrate (NO3) and nitrite (NO2) salts (soluble in water).
This is how atmospheric nitrogen is fixed/ converted into a usable form. Plants use these nitrates to make proteins.
Animals also eat plants to get proteins. Animal excreta and dead plants/animals again get converted to nitrates.
Denitrifying bacteria convert these nitrates back to atmospheric nitrogen. As a whole, % of N2 in the air remains constant.
WBBSE Chapter 8 Inorganic Chemistry In The Laboratory And In Industry Industrial Manufacture Of HCl, HNO3, And H2SO4
Manufacture of HCI (by Synthetic method): In the Castner-Kellner (commercial) process, caustic soda (NaOH) is manufactured by the electrolysis of aqueous sodium chloride (NaCI) solution. The by-products obtained in this process are H2 (g) and Cl2 (g).
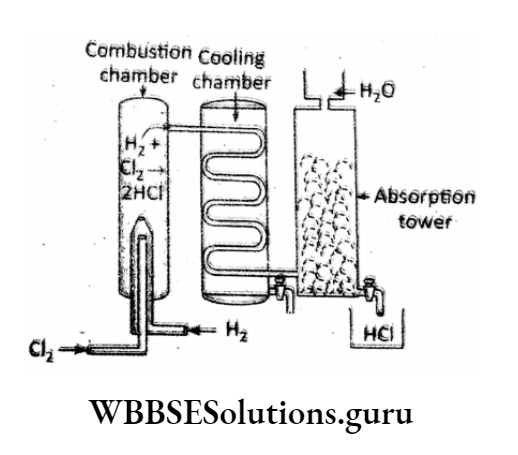
Industrially hydrogen chloride gas is manufactured by burning /direct combustion of equal volumes of H2 (g) and Cl2 (g) in a combustion chamber made of silica (SiO2).
⇒ \(\mathrm{H}_2(g)+\mathrm{Cl}_2(g) \rightarrow 2 \mathrm{HCl}(g)\)
The HCI (g) is passed through a cooling chamber. Then the cooled gas is allowed to absorb water. Finally, a saturated solution of hydrochloric acid is prepared.
⇒ \(\mathrm{HCl}(g) \stackrel{\mathrm{H}_2 \mathrm{O}}{\longrightarrow} \mathrm{HCl} \text { (aq.) }\left[\mathrm{H}^{+} \text {(aq.) }+\mathrm{Cl}^{-} \text {(aq.) }\right]\)
2. Manufacture of HNO3 (by Ostwald’s process):
The reaction\(\mathrm{NH}_3(g) \rightarrow \mathrm{HNO}_3 \text { (aq.) }\) takes place in 3 steps:
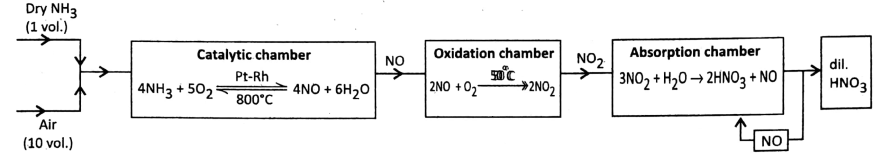
Step 1: Oxidation of NH3(g): A mixture of NH3 (g) and dry pure air in a 1:10 volume ratio is passed very fast over catalyst platinum-rhodium (90:10) gauze heated at about 800°C.
The time of contact is ~ 0-0014 seconds and NH3 (g) gets oxidized by O2 of air to produce nitric oxide vapor (NO).
The reaction is exothermic and reversible. Once the reaction starts, the heat evolves and continuous heating is not required.
⇒ \(4 \mathrm{NH}_3+5 \mathrm{O}_2 \stackrel{\mathrm{Pt}-\mathrm{Rh}}{\underset{800^{\circ} \mathrm{C}}{\rightleftharpoons}} 4 \mathrm{NO}(g)+6 \mathrm{H}_2 \mathrm{O}(g)+\text { Heat }\)
Step 2: Oxidation of NO vapor: Hot gaseous mixture (NO vapor + water vapor + excess O2) is cooled to 50°C and NO vapor is further oxidized by O2 sent in the oxidizing chamber to produce nitrogen dioxide gas (NO2).
⇒ \(2 \mathrm{NO}(g)+\mathrm{O}_2(g) \longrightarrow 2 \mathrm{NO}_2(g)\)
Step 3: Absorption of NO2(g): The absorption of NO2 (g) by water sprayed from the top of the absorption tower produces nitric acid (HNO3).
Initially, very dilute HNO3 is produced which is recycled to absorb, more and more NO (g) till 68% HNO3 is obtained.
Further concentration is done by distillation with a cone. H2SO4 at temperature ~120°C. This HN03 is almost 98% concentrated.
⇒ \(3 \mathrm{NO}_2(g)+\mathrm{H}_2 \mathrm{O}(I) \longrightarrow 2 \mathrm{HNO}_3 \text { (aq.) }+\mathrm{NO}(g)\)
⇒ \(\mathrm{HNO}_3(68 \%) \stackrel{+ \text { conc. } \mathrm{H}_2 \mathrm{SO}_4}{\underset{\text { Distillation }}{\longrightarrow}} \mathrm{HNO}_3(98 \%)\)
(3) Manufacture of H2SO4 (by Contact Process):
Step 1: Production of sulfur dioxide (SO2):
⇒ \(\mathrm{S}(\mathrm{s})+\mathrm{O}_2(g) \rightarrow \mathrm{SO}_2(g)\)
Or, \(4 \mathrm{FeS}_2(s)+11 \mathrm{O}_2(g) \longrightarrow 2 \mathrm{Fe}_2 \mathrm{O}_3(s)+8 \mathrm{SO}_2(g)\)
By heating sulfur or iron pyrites in the presence of air (O2) SO2 (g) is prepared [heating in the presence of excess air is called Roasting].
Step 2: Oxidation of sulfur dioxide(SO2) to sulphur trioxidfe (SO3): (most important step:)
\(\begin{aligned}& 2 \mathrm{SO}_2(g)+\mathrm{O}_2(g) \underset{\substack{450^{\circ} \mathrm{C} \\
2 \mathrm{~atm}}}{\stackrel{\mathrm{V}_2 \mathrm{O}_5}{\rightleftharpoons}} 2 \mathrm{SO}_3(g)+\text { Heat } \\
& \text { Excess air }\left(\mathrm{O}_2\right) \\
&
\end{aligned}\)
In the presence of a solid vanadium pentoxide catalyst (V2O5) at the optimum temperature of 450°C and high pressure -2 atm, pure and dust-free SO2 (g) is oxidized by dry pure O2 (g) to form SO3 (g).
Step 3: Absorption of SO3 (g): [SO3 is not diluted directly by adding water into \(\mathrm{SO}_3 \mathrm{O}\mathrm{SO}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{SO}_4\) (not done).
Because too much heat is evolved for SO3 and H2SO4 vapors form an acid mist.]
After cooling, SO3 (g) is dissolved in 98% concentrated H2SO4 sprayed from the top of the absorption chamber.
Fuming sulphuric acid or pyro-sulphuric acid (H2S2O7) is formed. Its commercial name is Oleum,
⇒ \(\left.\mathrm{SO}_3+\mathrm{H}_2 \mathrm{SO}_4 \text { ( } 98 \% \text { conc. }\right) \rightarrow \mathrm{H}_2 \mathrm{~S}_2 \mathrm{O}_7(l)\)
Then, after dilution with water, sulphuric acid of any desired concentration can be produced. \(\mathrm{H}_2 \mathrm{~S}_2 \mathrm{O}_7(I)+\mathrm{H}_2 \mathrm{O}(I) \rightarrow 2 \mathrm{H}_2 \mathrm{SO}_4 \text { (aq.) }\)
Note:
Oleum is a more powerful oxidizing agent than a con. H2SO4.
Solid crystals used in industrial manufacturing processes (Ostwald’s process/Contact Process) are usually taken in dust form not in bigger granules in order to get a greater surface area.
For it, the activity of catalysts/ rate of reaction increases because of greater adsorption.
